Integrated Rate Law Second Order Formula
Perform integrated rate law calculations for zero first and second order reactions.

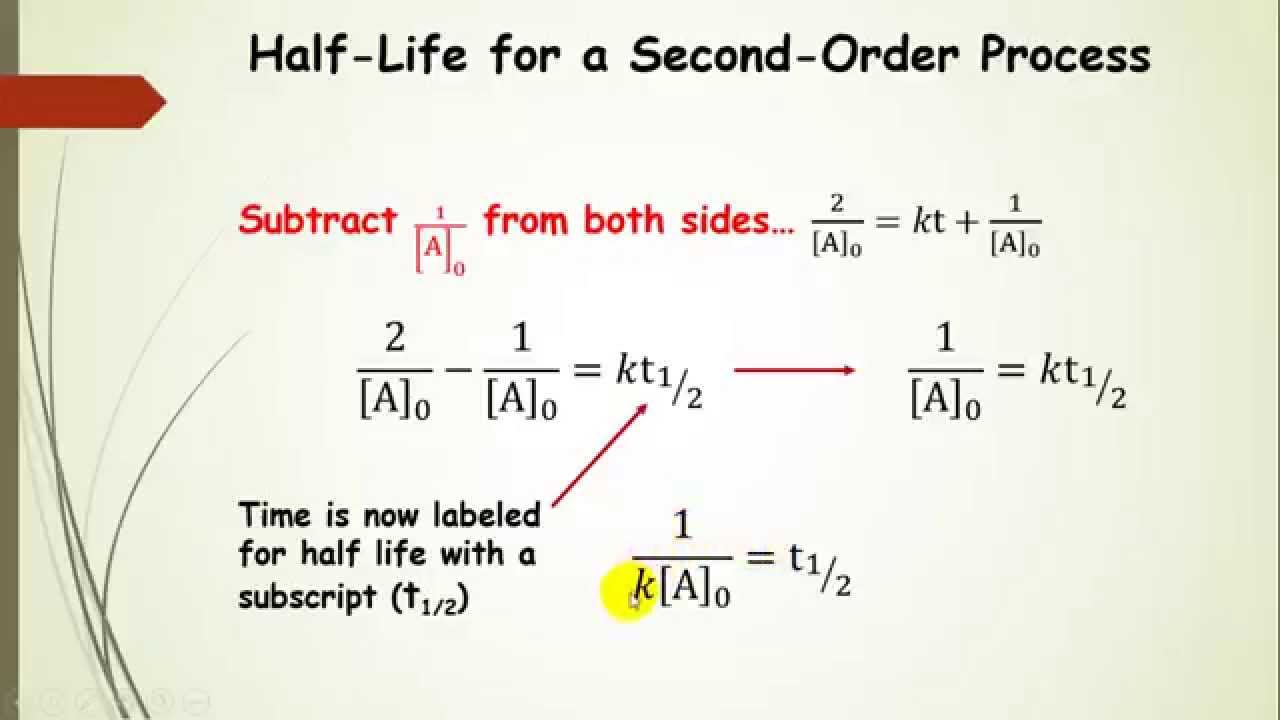
Integrated rate law second order formula. The rate law or rate equation for a chemical reaction is an. The second order rate equation has been reduced to a. The integrated rate equation for the. Using integrated rate laws.
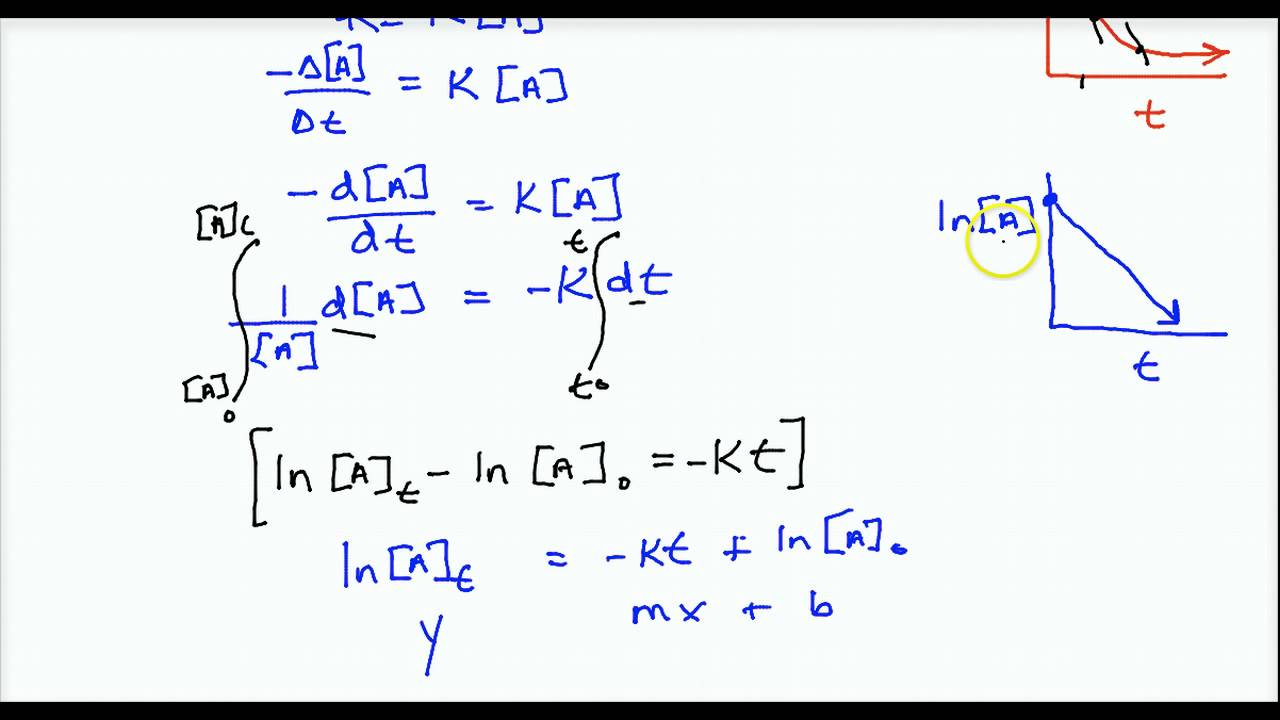
We use integrated rate laws and rate constants to relate concentrations and time. The rate law to use depends on the overall order of the. This chemistry video tutorial provides the equations and formulas needed to solve zero order first and second order integrated rate law problems including. In this lesson we will give the equation for the second order integrated rate law.
Well discuss its graph and then set up and solve a simple. This is the standard form for second order rate law and the integrated rate law will be the same as above. First order kinetics equation derivation integrated rate equation zero order reaction first order reaction second order reaction third order reaction. A problem in which we find the concentration of a reactant after a certain time the reaction has proceeded using the integrated rate law.
Explain the form and function of an integrated rate law.